- Matter
- Definitions for Components of Matter
- The Modern Atomic Theory
- Atom
- Structure of an atom
- Atomic Theory
- Thomson's Plum Pudding Model
- Rutherford’s Gold Foil Experiment
- Bohr's Model
- General features of the atom today
- Atomic Number (Z)
- Mass Number (A)
- Energy Levels
- Energy Levels in the Atom
- SUB ENERGY LEVEL OR ORBITALS
- The Outermost Shell
- Valency
- Isotopes
- Application of isotopes
- Isobars
Matter
Anything that has mass and volume
Occur in three physical states:
- Solid (Volume and shape are fixed) (eg, Wood, Ice, Playstation remote control)
- Liquid (Volume, but not shape, is fixed) (eg, H2O, Alcohol, oil)
- Gas (Volume and shape are not fixed) (eg, CO2 , O2 ,NO2)
Definitions for Components of Matter
Classified into three types:
- Element
- Compound
- Mixture
1.Element
The simplest type of substance with unique physical and chemical properties. An element consists of only one type of atom. It cannot be broken down into any simpler substances by physical or chemical means
2.Compound
A substance composed of two or more elements which are chemically combined. (e.g. NH3)
3.Mixture
a group of two or more elements and/or compounds that are physically intermingled.(e.g. softdrinks, cement, milk)
Molecule – a structure that consists of two or more atoms that are chemically bound together and thus behaves as an independent unit.
Substance – a form of matter that has a definite (constant) composition and distinct properties such as appearance, smell, taste, and others. Eg. Water, gold, table sugar and oxygen.
The Modern Atomic Theory
All matter is composed of atoms
Atoms of one element cannot be converted into atoms of another element in a chemical reaction, but can in a nuclear reaction
All atoms of an element have the same number of protons and electrons, which determine the chemical behavior of the element
Compounds are formed by chemical combination of two or more elements in specific ratio
Atom
- Electrically neutral
- Spherical
- Consist of a positively charged nucleus, around which negatively charged electrons (e-) are found
What is the force that holds electrons around the atomic nucleus?
- Atom was discovered by John Dalton. He proposed the famous atomic theory in 1807.
- Atoms are fundamental unit of matter.
- The existence of different kinds of matter is due to different atoms constituting them.
- A major challenge before the scientists at the end of the 19th century was to reveal the structure of the atom as well as to explain its important properties.
- Many scientists worked hard and proposed many models for the atom, here we are going to learn about the structure of an atom .
Structure of an atom
The discovery of the two fundamental particles (electrons and protons) inside the atoms led to the failure of the aspect of Daltons atomic theory.
For explaining the arrangement of electrons and protons in an atom, many scientists proposed various atomic models.
Atomic Theory
- All matter is made up of atoms.
- Atoms of an element are identical.
- Each element has different atoms.
- Atoms can engage in a chemical reactions.
- Atoms can neither be created nor be destroyed.
- Atoms are indivisible.
Thomson’s Plum Pudding Model
In 1897 the English scientist JJ Thomson provided the first hint that an atom is made of even smaller particles.
He proposed a model of the atom that is sometimes called the “Plum Pudding” model.
Atoms were made from a positively charged sphere with negatively charged electrons embedded in it, like raisins in a pudding.
The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically stable
Rutherford’s Gold Foil Experiment
In 1908, the English physicist Ernest Rutherford was hard at work on an experiment that seemed to have little to do with unraveling the
mysteries of the atomic structure.
Rutherford’s experiment Involved firing a stream of tiny positively charged alpha (α) particles at a thin sheet of gold foil.
The expected result was that the α- particles would be deflected by the sub- atomic particles in gold atoms. Since α – particles were much heavier than protons, he did not expect to see larger deflections
Most of the positively charged “bullets” passed right through the gold atoms
in the sheet of gold foil without changing course at all.
Some of the positively charged “bullets,” however, did bounce away from the gold sheet as if they had hit something solid. He knew that positive charges
repel positive charges.
This could only mean that the gold atoms in the sheet were mostly open space. Atoms were not a pudding filled with a positively charged material.
Rutherford concluded that an atom had a small, dense, positively charged center that repelled his positively charged “bullets.”
He called the center of the atom the “nucleus”
The nucleus is tiny compared to the atom as a whole
Bohr’s Model
In 1913, the Danish scientist Niels Bohr proposed an improvement. In his model, he placed each electron in a specific energy level.
Following Rutherford’s planetary model of the atom, it was realized that the attraction between the electrons and the protons should make the atom unstable
Bohr proposed a model in which the electrons would stably occupy fixed orbits, as long as these orbits had special discrete locations
General features of the atom today
The atom is an electrically neutral, spherical entity composed of a positively charged central nucleus surrounded by one or more negatively charge electrons.
The atomic nucleus consists of protons and neutron
Atomic Number (Z)
Number of p+ in the atomic nucleus of an element
Number of e- in an atom of an element
All atoms of an element have the same Z
Different elements will have different Z

Mass Number (A)
Total number of protons and neutrons in the atomic nucleus of an element

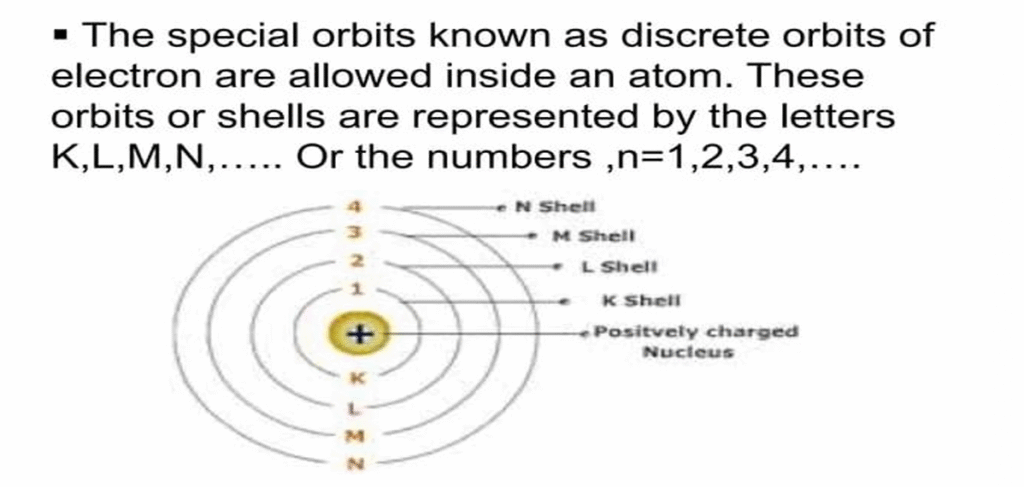
Energy Levels
- ENERGY LEVELS (SHELLS)
- Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found.
- Energy levels are a little like the steps of a staircase.
- You can stand on one step or another but not in between the steps. The same goes for electrons.
- They can occupy one energy level or another but not the space between energy levels.
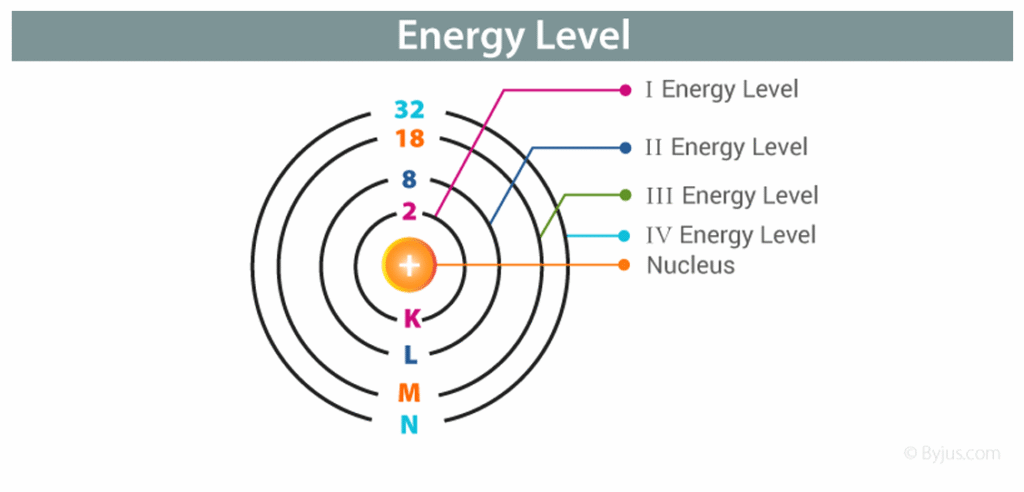
Energy Levels in the Atom
- As we considered, the electrons in the electrons in the atom can only occupy quantized orbits, i.e.,
- ENERGY LEVELS or SHELLS.
- All electrons prefer top be in the lowest energy level .
- That is why an electron at a higher energy level eventually falls in a lower level resulting in the release of a photon.
- There is a potential well in the atom where the top of the well is at zero potential and the other energy levels are at negative potential. The ground level will be at the lowest potential energy
SUB ENERGY LEVEL OR ORBITALS
- An orbital is a volume of space within an atom where an electron is most likely to be found.
- The maximum number depends on the number of orbitals at a given energy level.
- Electrons belonging to the same energy level may differ in their energy.
- So, energy level have been divided into sub energy levels as S,P,D,F (sharp, principal, diffuse and fundamental).
- Regardless of its shape, each orbital can hold a maximum of two electrons.
- Energy level I has just one orbital, so two electrons will fill this energy level.
- Energy level II has four orbitals, so it takes eight electrons to fill this energy level.
- According to Schrodinger, electrons are present in orbitals in the space around the nucleus in three dimensions where possibility of finding the electrons is maximum
The Outermost Shell
- Electrons in the outermost energy level of an atom have a special significance.
- These electrons are called valence electrons, and they determine many of the properties of an atom.
- An atom is most stable if its outermost energy level contains as many electrons as it can hold.
- Consider the elements fluorine and lithium, Fluorine has seven of eight possible electrons in its outermost energy level, which is energy level II.
- It would be more stable if it had one more electron because this would fill its outermost energy level.
- Lithium, on the other hand, has just one of eight possible electrons in its outermost energy level (also energy level II).
- It would be more stable if it had one less electron because it would have a full outer energy level (now energy level I)
Valency
- The outermost shell of an atom is known as its valence shell.
- The electrons present in the outermost shell of an atom are known as the valence electrons
- The Valency of an element may be defined as the combining capacity of its atoms with atoms of other elements in order to acquire octet configuration.
- For eg; The valency of hydrogen is 1.
Isotopes
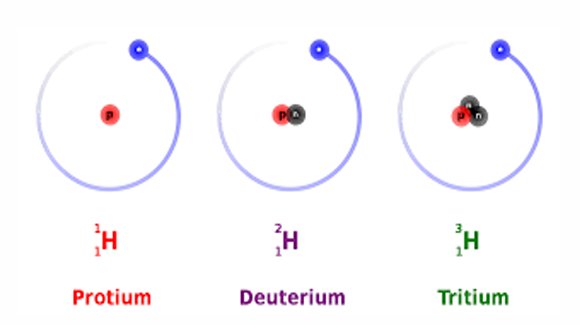
Isotopes are atoms of same element, which have different mass numbers but same atomic number.
Their chemical properties are similar but physical properties are different
Application of isotopes
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotopes of cobalt is used in the treatment of cancer.
- An isotope of iodine is used in the treatment of goitre
Isobars
Atoms of different elements with different atomic numbers ,which have the same mass number are known as isobars.
Examples of isobars are: calcium(z=20)and argon(z=18).their mass number is 40 u.

